As part of the Foundation for the National Institutes of Health (FNIH) Accelerating Medicines Partnership® Bespoke Gene Therapy Consortium (AMP® BGTC), Nemours Children’s Health has been chosen to conduct a first-of-its-kind gene therapy clinical trial for Morquio A syndrome.
Nemours Children’s will work collaboratively with the FNIH AMP® BGTC, a public-private partnership between the National Institutes of Health (NIH), U.S. Food and Drug Administration (FDA), biopharmaceutical and life science companies, and non-profit and other organizations, to help speed the development and delivery of customized or ‘bespoke’ gene therapies. Eight genetic diseases were selected for clinical trials, which will take place across the country. Nemours Children’s was chosen for their Morquio A project proposal and will also be the site of the clinical trial.
Gene therapy is the promise of the future for many patients with rare disorders. Much of my research has been focused on Morquio A syndrome over the past 35 years. It is very exciting to see the hard work result in a promising clinical trial that could be life-changing for this patient population.”
Shunji Tomatsu, MD, PhD, Principal Investigator, Nemours Children’s Health, Delaware
Nemours Children’s Health is internationally renowned for diagnosing and treating Morquio A syndrome, a rare skeletal dysplasia, affecting 1 in 200,000, caused by an inherited gene mutation. Leading pediatric orthopedic researchers, surgeons, and geneticists at Nemours have established a nationally recognized skeletal dysplasia program. Dr. Tomatsu has held NIH grant funding for Morquio syndrome since 2010. Furthermore in 2021, additional NIH funding was established to develop a complete natural history for Morquio A syndrome. Nemours researchers have also been studying new and innovative treatments to help cure this genetic disorder that causes serious complications including cervical spinal cord compression, short stature, flat feet, difficulty walking, tracheal obstruction, hearing loss, and heart valvular disease.
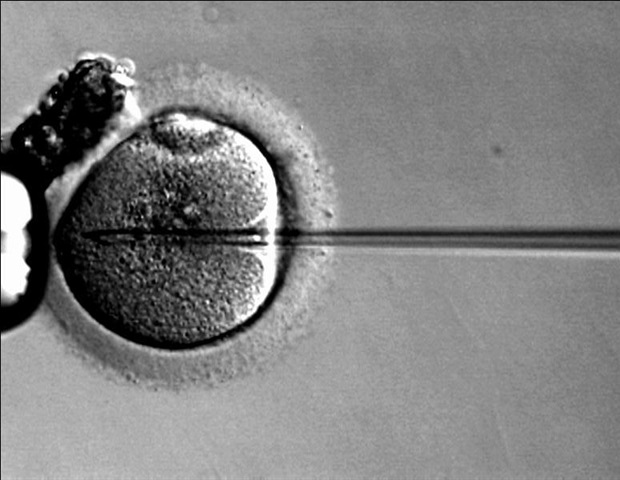
Most recently, Dr. Tomatsu and his colleagues identified through murine models that Adeno-associated virus (AAV) gene therapy could be the answer for these patients. With AAV gene therapy, patients are given a one-time infusion which consists of a viral vector containing the correct gene sequence that can transfer genetic material safely into tissues and cells.
“The ultimate goal of our work is to help our patients. With the knowledge we gain during this trial, we believe Nemours Children’s Health will be able to offer Morquio patients the newest and most innovative therapies available,” said Stuart Mackenzie, MD, Orthopedic Surgeon, Director of the Skeletal Dysplasia Clinic, Nemours Children’s Health, Delaware. “Through this specialized public and private partnership with FNIH AMP, we are able to help realize our vision to create the healthiest generations of children.”
For the remainder of the year, FNIH and Nemours will focus on refining the treatment protocol, planning, and hiring additional professional staff. Nemours Children’s aims to start enrollment for the clinical trial in 2024.
Nemours Children’s would also like to acknowledge the many other colleagues that have been instrumental in developing this clinical trial including Michael B. Bober, MD, Medical Director of Orthogenetics, Kimberly Kipner, Clinical Research Coordinator, Allison Bradford, Clinical Research Assistant and Lan He, Research Lab Manager.
Private philanthropy in support of novel research efforts such as this is critically important. There have been several generous individuals and families who have provided funding throughout the course of this work. In particular, Nemours would like to acknowledge the dedicated fundraising efforts of A Cure for Robert, Inc. (Rooting for Robert) and the generous support of the Angelo R. Cali & Mary V. Cali Family Foundation, Inc. and Morquio Community, Inc.